This automated data science-driven and scalable subscription service eliminates the manual, resource intensive and error-prone efforts that can interfere with achieving adherence and replaces them with a streamlined approach to optimizing hazardous drugs handling– and protecting your business.
JOIN US FOR AN UPCOMING HEALTHCARE EVENT
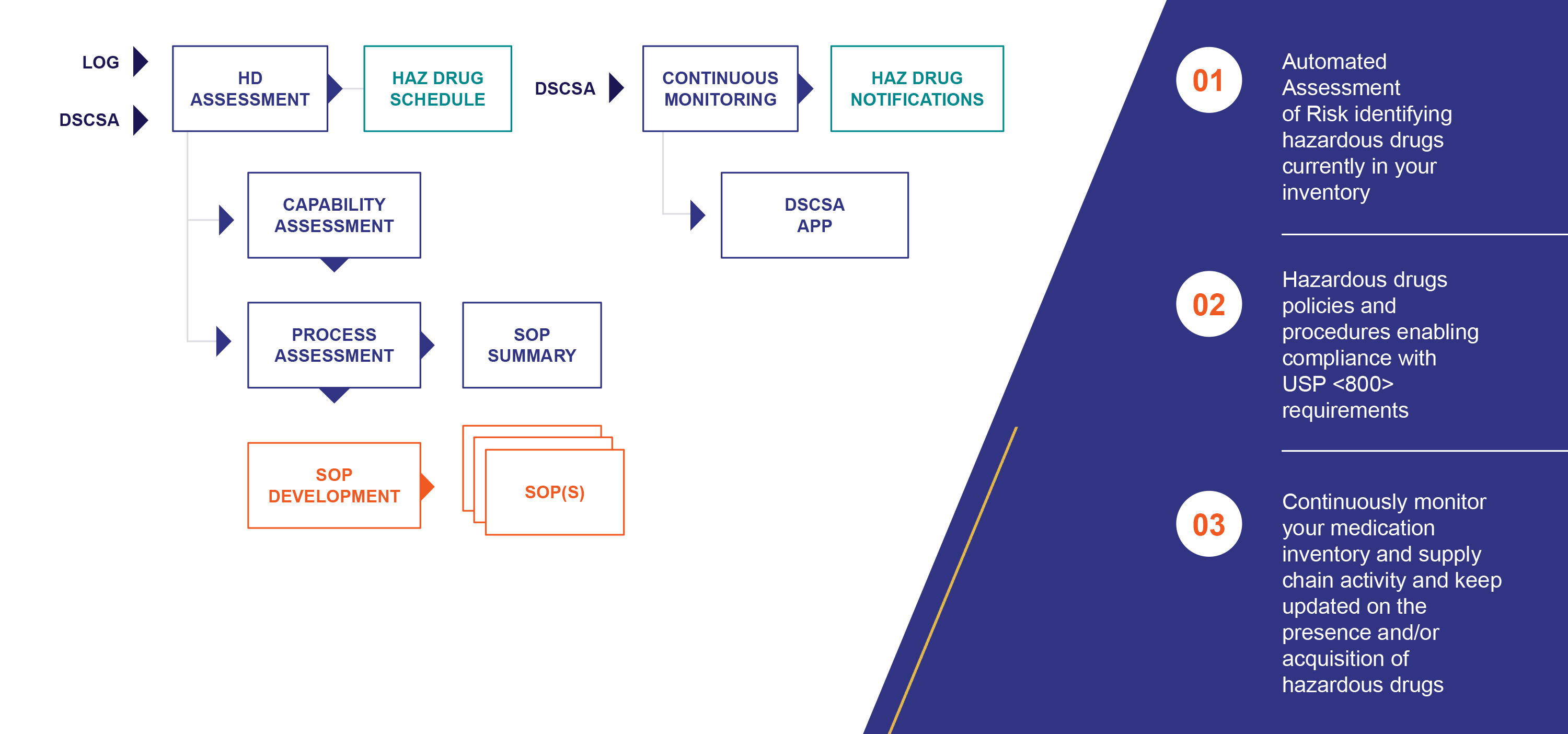
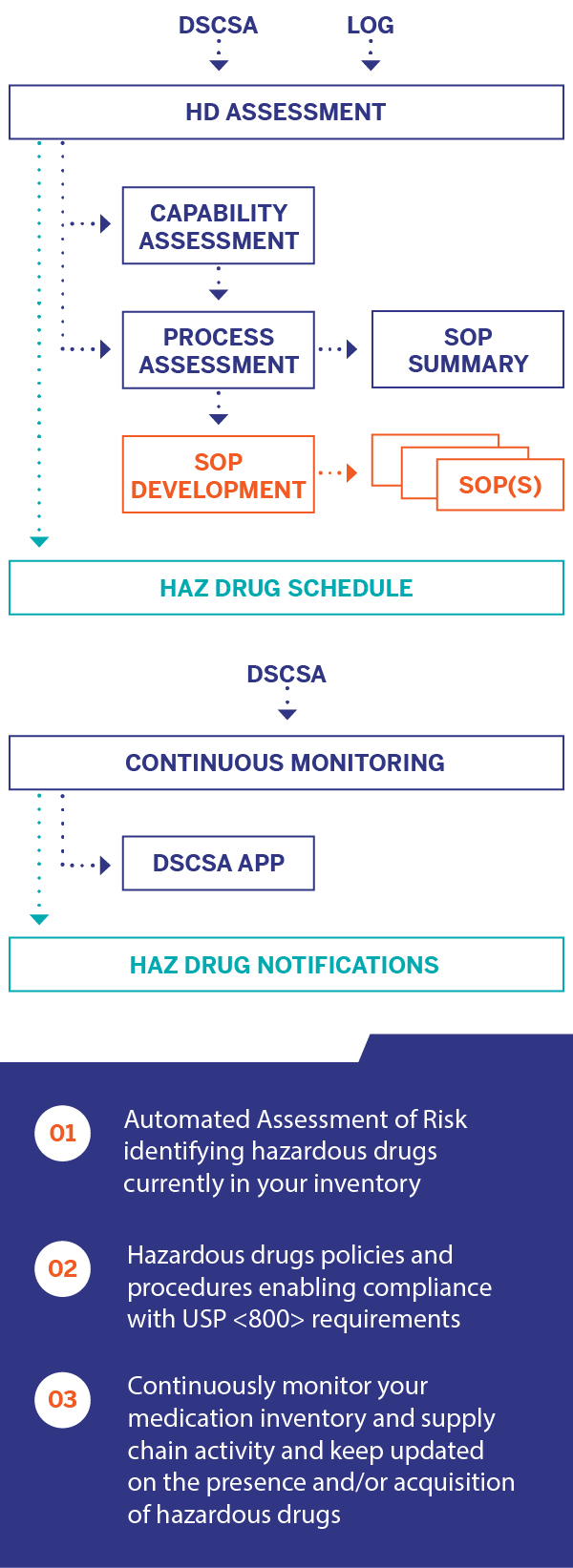
As of December 1, 2019, USP <800> became enforceable. One of the biggest challenges to complying with this regulation is the requirement of annually performing an Assessment of Risk that identifies the drug products in your formulary that NIOSH (National Institute of Occupational Safety and Health) has classified as a hazardous drug.
USP General Chapter <800> provides standards for the safe handling of hazardous drugs in order to minimize the risk of exposure to healthcare personnel, patients and the environment. Instituting these standards promises real benefit for many– along with a significant burden for pharmacies. That burden includes establishing new procedures for the receipt, storage, administration and work practices to minimize the risk of exposure to hazardous drugs. It’s a lot to ask when you’re focused on patient care– and that’s why Inmar is offering our USP <800> Solution.
Inmar’s USP <800> Solution Overview: